- solution
- Product center
- event
- Agent network
- Serve
- about us
- contact us
- 中文
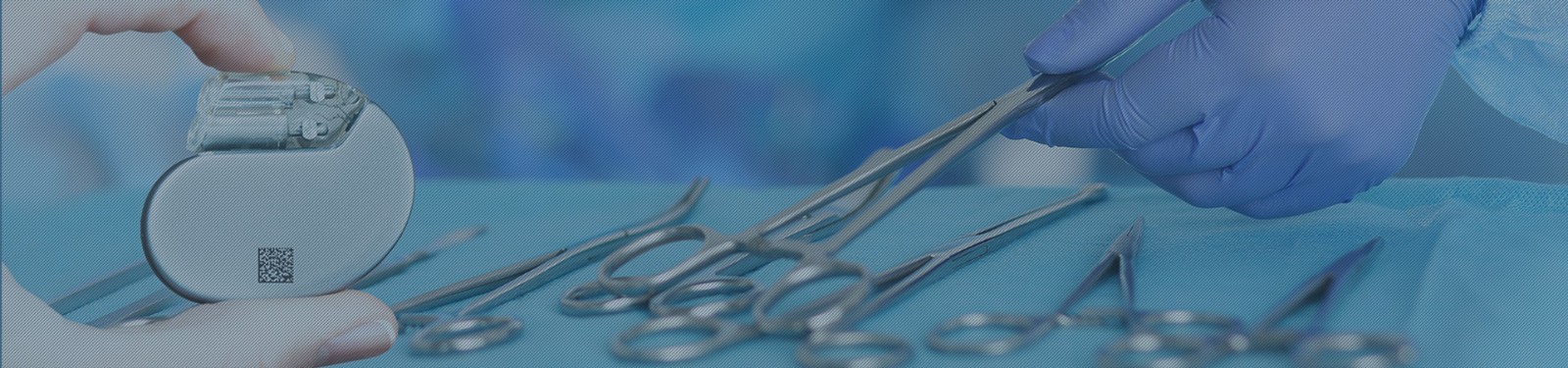
"Live marking expert" explains UDI (UNIQUE DEVICE IDENTIFICATION)
EU EU medical equipment regulations (MDR) 2017/745 requires manufacturers, distributors, importers, and EU representatives of medical devices or their packages to clearly mark their products from May 26, 2021. Therefore, medical devices will be completely traceable to the manufacturer to provide users with safety in patients who require EU requirements.
All participants have equal binding power based on standards and regulations adopted by medical device regulations. To this end, the product identification required by the medical device is issued by one of the four distribution agencies - GS1, HIBCC, ICCBBA and INFORMATIONSTELLE FÜR ARZNEISPEZIALITÄTEN.
Since May 2022, all medical devices will be stored in the Eudamed database in the European Union along with their main data and "unique device identification" (UDI).
"Live marking expert" explains UDI (UNIQUE DEVICE IDENTIFICATION)
EU EU medical equipment regulations (MDR) 2017/745 requires manufacturers, distributors, importers, and EU representatives of medical devices or their packages to clearly mark their products from May 26, 2021. Therefore, medical devices will be completely traceable to the manufacturer to provide users with safety in patients who require EU requirements.
All participants have equal binding power based on standards and regulations adopted by medical device regulations. To this end, the product identification required by the medical device is issued by one of the four distribution agencies - GS1, HIBCC, ICCBBA and INFORMATIONSTELLE FÜR ARZNEISPEZIALITÄTEN.
The product logo consists of two parts:








